Coccidioides immitis (Etiology of coccididiomycosis)
My name is Olanrewaju ADEYEMI, a
graduate student in Biological sciences at Western Illinois University. I
developed this blog to make people aware of medical importance Fungi. Coccidioides immitis was used as the
subject organism and I hope this blog will increase knowledge about medical
mycology.
GENERAL
DESCRIPTION
Taxonomic
classification:
Kingdom:
Fungi
Phylum: Ascomycota
Class: Eurotimycetes
Order: Onygenales
Family: Onygenaceae
Genus:
Coccidioides
Species: immits.
(www.mushroomobserver.org, 2009).
Coccidioides immitis is a dimorphic fungus that has
the ability to exist in two distinct forms. It lives primarily in the soil
(Davis et al. 1942). C. immitis
colonies grow at a very fast rate compared to other medically important fungal
at either 25 or 37°C and on Sabouraud Dextrose Agar. The colonies are moist,
and grayish at first, later they produce white and cottony aerial mycelium.
After a long time, colonies become tan to brown in color (Drutz, D., and M.
Huppert, 1896). Coccidiosis immitis
belongs to a class of human pathogenic fungi and exist in both saprophytic and
parasitic forms. In the saprophytic form, it occurs as a mould with septate hyphae
looking like barrel. As a parasite, the arthroconidia (spores) are released
from the hyphae and transformed into spherules (Fig1). The spherules grow and
also divide intrinsically, forming smaller structures called endospores inside
the host (Drutz, and Hupper, 1983).The parasitic form is known to cause a wide
spectrum of aliments, varying from a subtle illness to severe pulmonary
manifestations or disseminated disease.
(Fig.1) A
photomicrograph showing scattered strains of arthroconidia of the fungus Coccidioides immitis (https://www.google.com/search?q=coccidioides+immitis&source=lnms&tbm=isch&sa=X&ved=0CAcQ_AUoAWoVChMIt8HljY7oyAIVhygeCh3EyQwb&biw=1525&bih=712&dpr=0.9#tbm=isch&q=coccidioides+immitis+mould+form&imgrc=pKOVWchSbZaVPM%3A).
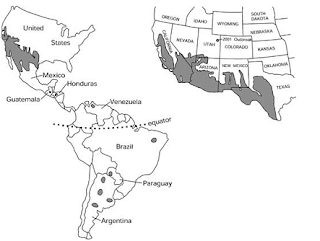
Geographic distribution
Commonly
found in arid to semiarid regions of the southern San Joaquin Valley,
California, which is part of the Lower Sonoran Lifezone (Maddy et al.
1957). Also found in New Mexico, Nevada,
Texas, and Utah; and in Central and South America in Argentina, Brazil,
Colombia, Guatemala, Honduras, Mexico, Nicaragua, Paraguay, and Venezuela
(Frederick et al. 2013). The geographic range for Coccidioides
immitis has been described based on epidemiologic studies of persons
infected and diagnosed with coccidioidomycosis. Edwards and Palmer in 1957 used
skin testing to map the distribution of Coccidioides in the USA. The
results of this and similar studies established that the south-central valley
of California and the deserts of southern Arizona, were the most highly endemic
for Coccidioides (Beadenkopf et al. 1949) (Fig 2). Presently, it is known
that Coccidioides spp. are endemic to specific regions in the Western
Hemisphere, primarily those located between the north and south 40° latitudes (Burt
A et al. 1997).
(Fig2)
Coccidioides in
North, Central, and South America. Endemic areas are shown in gray
Habitat
and life cycle
Research
has shown that factors like physical, chemical and biological, affect the
growth of Coccidioides (Fiese 1958, Sorensen 1964). Important factors
include: amount and timing of rainfall and moisture, soil humidity, soil
temperature, soil texture, alkalinity, salinity, organic content of soils,
amount of sunlight and ultraviolet light.
Approximately
100,000 primary coccidioidal infections occur in humans per year in the areas
of endemicity in the United States (Galgiani et al. 1999).
Increment of the disease have been recorded in California and Arizona, which
may be partially due to the rapid immigration of previously unexposed persons
from states outside the endemic areas (Ampel et al. 1998). Generally, diagnosis
in patients who have symptoms is established by serological diagnosis combined
with patient history. Coccidioidal skin test antigen was a useful adjunct in
the diagnosis decades ago, but it became unavailable in the 1980s (Smith CE et al. 1951).
Life Cycle: (fig 3).The organism follows the saprophytic
cycle in the soil and the parasitic cycle
in vertebrates. The saprophytic cycle starts in the soil with arthrospores
(mould form) that develop into mycelium. The
mycelium then matures and forms alternating spores within itself. The
arthrospores are then released, and germinate back into mycelia. The parasitic
cycle which occur in vertebrates involves the inhalation of the arthrospores by
animals which then mature, at maturation, form spherules filled with
uninucleated endospores. C. immitis is unique because it produces
spherules containing endospores in tissue, and hyphae at 25°C. In addition,
since C. immitis is an anaerobic organism, spherules produce rapidly
in presence of CO2. Increased temperature and nutrition are also
important for the production of sporulating spherules
(http://www.ppdictionary.com/mycology/immitis.htm).
(Fig3).
Life Cycle of Coccidioides immitis
(Illustration: Michael Borjon/The Bakersfield Californian). http://www.ncbi.nlm.nih.gov/pmc/articles/PMC545195/figure/pmed-0020002-g002/
Clinical
manifestations and diagnosis
About
60% of infections by this fungus results in asymptomatic infections (Smith et al. 1946).
Symptoms in the 40% remaining can be a primary, a benign, a pulmonary
infection (commonly known as “Valley Fever”) to a progressive pulmonary or
extra pulmonary disease involving different parts of the body including central
nervous system. Most patients with primary disease are self-limiting and retain
lifelong immunity to future exogenous infection. Chronic and disseminated
disease is known to occur in up to 5% of infected individuals, with
comparatively more cases occurring in older individuals and in males (Ampel et
al.1998). The most dangerous form of the disease is in the central nervous
system, which occur in about 0.15%–0.75% of extrapulmonary coccidioidomycosis
cases and requires treatment throughout lifetime (Cortez
et al. 2003). In a tuberculosis endemic area, both diseases may occur at
the same time. Coccidioidomycosis can be misdiagnosed for tuberculosis because
both disease share common epidemiological, clinical, radiographic, and even
histopathological features (Castañeda-Godoy; et al. 2002).
CASE STUDY 1
A 72-year-old man in the outpatient
unit of a hospital presented with a four-month history of progressive dyspnea,
hemoptysis and weight loss. The patient was a resident of southwestern Ontario
and had been travelling to Arizona annually for six years. He had a significant
smoking history. Three years before, he had a left upper lobe cavityof the lung
lesion diagnosed as a poorly differentiated bronchogenic carcinoma with
coexistent blastomycosis. For treatment, a left upper lobectomy was performed
and itraconazole was given for six months.
The patient’s most recent chest x-ray
showed left pleural thickening, unchanged from three years previously. A
presumptive diagnosis of recurrent bronchogenic carcinoma and/or blastomycosis
was made, and the patient underwent investigation with fibre optic bronchoscopy
(Lee et al. 2008).
On bronchoscopy, abnormal tissue was
found invading the left lower lobe. Bronchoalveolar lavage (BAL), along with
brushings and a biopsy, was performed. The bronchial washing smear showed
atypical cells, interpreted as a poorly differentiated nonsmall cell carcinoma
along with occasional organisms identified as Blastomyces species. The
biopsy specimen showed atypical squamous epithelium and fungal organisms
compatible with Blastomyces species, but no malignant cells were
detected. Direct microscopy of the tissue biopsy, which was performed in the
hospital microbiology laboratory using a calcofluor white preparation, showed
the presence of a few spherules, suggestive of C. immitis. A culture
of the specimen grew C. immitis. Molecular technique was further used
to confirm that the isolate was C. immitis (Lee et al. 2008).
CASE STUDY 2
A 51-year-old man presented with right-sided pleuritic chest
pain and cough. He just returned from a camping trip to the Grand Canyon
(Arizona, USA). He was misdiagnosed and treated with an oral antibiotic for
community-acquired pneumonia, based on his symptoms and an infiltrate on chest
radiography. At follow-up, he complained of cough (only occasionally productive
of clear sputum) and had no episodes of hemoptysis. He experienced intermittent
fevers and chills, but no other systemic complaints or weight loss. He was
previously well and on no regular medications. Baseline blood work including
complete blood counts, electrolytes, creatinine and liver enzymes were normal (Lee et al. 2008).
A repeat chest radiography and high-resolution CT scan
(computed tomography) of the chest showed an opacity in the periphery of the
right upper lobe. There were no calcifications or cavitations within the
lesion. He underwent bronchoscopy, with BAL, mediastinoscopy and biopsy. The
organisms seen on the BAL smear were interpreted by a pathologist to be
consistent with Blastomyces species and a culture of the specimen grew C.
immitis (Lee et
al. 2008).
REFERENCES
1
Davis,
B. L., Jr., Smith, R. T. &Smith, C. E. An
epidemic of coccidioidal infection (coccidioidomycosis). J. Am. Med. Assoc.
118:1182–1186. (1942)
Drutz,
D. J., and M. Huppert. Coccidioidomycosis:
factors affecting the host-parasite interaction. J. Infect. Dis. 147:
372-390 (1983)
3 Edwards
PQ, Palmer CE. Prevalence of sensitivity to coccidioidin, with special
reference to specific and nonspecific reactions to coccidioidin and to
histoplasmin. Dis Chest. 1957;31(1):35–60.
4
Beadenkopf WG, Loosli CG, et al.
Tuberculin, coccidioidin, and histoplasmin sensitivity in relation to pulmonary
calcifications; a survey among 6,000 students at the University of Chicago. Public
Health Rep. 1949;64(1):17–32.
[PubMed]
Burt
A, Dechairo BM, Koenig GL, Carter DA, White TJ, Taylor JW. Molecular markers
reveal differentiation among isolates of Coccidioides immitis from California,
Arizona and Texas. Mol Ecol. 1997;6(8):781–786
6
Drutz,
D. J., and M. Huppert. Coccidioidomycosis: factors affecting the host-parasite
interaction. (http://www.ncbi.nlm.nih.gov/sites
/entrez?cmd=Retrieve&db=PubMed&list_uids=6300253&
dopt=AbstractPlus) J. Infect. Dis. 147: 372-390 (1983).
7
Maddy
K. 1957. Ecological factors of the geographic distribution of Coccidioides
immitis. J Am Vet Med Ass 130:475–476.
immitis. J Am Vet Med Ass 130:475–476.
8
Frederick
S. Fisher, Mark W. Bultman, Suzanne M.Johnson, Demosthenes
Pappagianis, and Erik Zaborsky Coccidioides Niches
and Habitat Parameters in the Southwestern United StatesAnn. N.Y. Acad. Sci.
1111: 47–72 (2007).
Fiese,
M.J. 1958. Coccidioidomycosis. Charles C Thomas. Springfield, IL.
1Sorensen,
R.H. 1964. Survival characteristics of mycelia and spherules of Coccidioides
immitis in a simulated natural environment. Am. J. HyG. 80: 275–285.
1
Smith CE, Beard RR, Whiting EG,
Rosenberger HG. Varieties of coccidioidal infection in relation to the
epidemiology and control of the diseases. Am J
Public Health. 1946;36:1394–1402. [PMC free article] [PubMed]
Ampel NM, Mosely DG, England B,
Vertz PD, Komatsu K, et al. Coccidioidomycosis in Arizona: Increase in
incidence from 1990 to 1995. Clin Infect Dis. 1998;27:1528–1530. [PubMed]
1
Cortez KJ, Walsh TJ, Bennett JE.
Successful treatment of coccidioidal meningitis with voriconazole. Clin
Infect Dis. 2003;36:1619–1622. [PubMed]
1
Castañeda-Godoy R,
Laniado-Laborín R. Coexistencia de tuberculosis y coccidioidomicosis.
Presentación de dos casos clínicos. Rev Inst Nal Enf Resp Mex. 2002;15:98–101.
1
Galgiani JN. Coccidioidomycosis:
A regional disease of national importance; Rethinking our approaches to its
control. Ann
Intern Med. 1999;130:293–300. [PubMed]
1
Smith CE. Diagnosis of
pulmonary coccidioidal infections. Calif Med. 1951;75:385–391.
[PMC free article] [PubMed]
Lee,
C. H., Wilcox, L., Chorneyko, K., & McIvor, A. (2008). Coccidioides
Immitis: Two Cases of Misidentified Mycosis. Canadian Respiratory
Journal : Journal of the Canadian Thoracic Society, 15(7), 377–379.